Charge once, power long: ultrasonic solution for implantable devices
GA, UNITED STATES, June 16, 2025 /EINPresswire.com/ -- A new study has unveiled a new method for wirelessly powering implantable medical devices (IMDs) using a pre-charged capacitive micromachined ultrasonic transducer (CMUT). Unlike traditional systems that require a continuous external direct current (DC) bias, this novel CMUT is equipped with an internal charge storage capacitor, allowing it to operate in a constant-charge mode. With an impressive energy conversion efficiency of up to 29.7% and the ability to deliver 10.1 mW of power—well above typical IMD requirements—the system maintains stable charge levels for over two years. This breakthrough promises a safer, smaller, and more durable energy source for next-generation biomedical implants.
Modern implantable medical devices (IMDs)—such as pacemakers, defibrillators, and neurostimulators—are constrained by their reliance on onboard batteries, which limit device longevity and increase the need for surgical replacements. Wireless power transfer methods, including inductive, capacitive, and electromagnetic coupling, have shown promise but are hindered by poor tissue penetration, alignment issues, and safety concerns. Ultrasound-based energy delivery has emerged as a compelling alternative due to its deep tissue reach and favorable safety profile. As the lead-based piezoelectric materials raise biocompatibility concerns for implantable devices, micromachined transducers attract greater attention for this application. However, most existing capacitive micromachined ultrasonic transducer (CMUT) systems still depend on external direct current (DC) bias, complicating their use. These challenges point to a pressing need for a self-sustaining, efficient ultrasonic power solution.
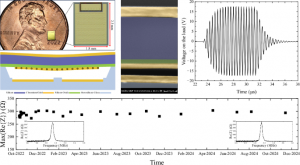
Responding to this need, engineers at North Carolina State University have developed a CMUT device that sidesteps the limitations of conventional systems. Reported in Microsystems & Nanoengineering on April 23, 2025, their CMUT design integrates a built-in charge storage capacitor and functions without the need for continuous external bias. Once pre-charged before implantation, the device retains its charge for years. In testing, it delivered a maximum output of 10.1 mW with a peak energy conversion efficiency of 29.7%, setting new records for ultrasonic wireless power in biomedical applications.
The heart of the innovation lies in the CMUT's architecture: a floating electrode sandwiched between two fixed electrodes. Prior to implantation, a DC bias and ultrasonic pressure bring the floating and bottom electrodes into contact, allowing controlled charge transfer. Once charged, the device converts incoming ultrasonic waves into electrical power without further external input. Laboratory tests confirmed strong performance, with stable operation at 2.45 MHz and no performance degradation over time. Crucially, all testing parameters remained within FDA safety limits. The design's key advantages—including vacuum encapsulation, charge-preserving insulation, and mechanical safeguards—enabled robust, leakage-free power delivery ideally suited for long-term use in IMDs.
"This technology marks a substantial step forward in the field of implantable power systems," said Dr. Ömer Oralkan, senior author of the study. "By eliminating the need for external bias and demonstrating years-long charge retention, we've made ultrasonic wireless power transfer not only more efficient but also more practical. The CMUT structure we developed is scalable, biocompatible, and can meet the power needs of diverse medical devices, from pacemakers to drug pumps."
Looking ahead, the researchers envision the development of 2D CMUT arrays to address alignment challenges and integrate with custom-designed circuits to increase power output. These advances could enable a new generation of compact, battery-free implants that operate safely and reliably for years. Beyond enhancing patient comfort and reducing surgical risk, the approach could redefine how clinicians and engineers design and power the next wave of bioelectronic devices.
References
DOI
10.1038/s41378-025-00902-w
Original Source URL
https://doi.org/10.1038/s41378-025-00902-w
Funding information
This work was supported by the NSF under Grant 1160483 as part of a Centre-to-Center collaborative partnership with NUI Galway, Ireland and Queen’s University, Belfast, Northern Ireland. The authors would like to thank Ali Önder Biliroğlu for his help with experimental setups. This work was performed in part at the NCSU Nanofabrication Facility (NNF), and Duke University Shared Materials Instrumentation Facility (SMIF), all of which are members of the North Carolina Research Triangle Nanotechnology Network (RTNN), which is supported by the National Science Foundation (Grant ECCS-2025064) as part of the National Nanotechnology Coordinated Infrastructure (NNCI).
Lucy Wang
BioDesign Research
email us here
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.